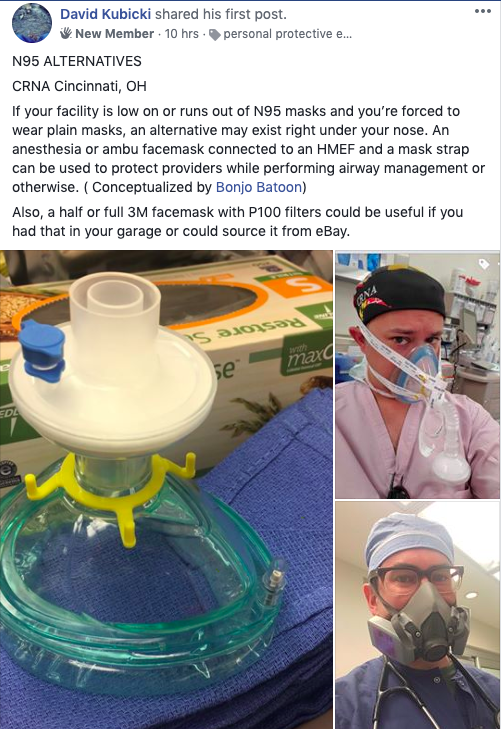
PLEASE NOTE, DUE TO MANY INCONSISTENCIES IN THE INFORMATION AVAILABLE ABOUT THE VIRUS (INCLUDING INFORMATION FROM THE OFFICIAL SOURCES AND FROM THE SCIENTIFIC STUDIES), I HAVE DECIDED NOT TO SHARE ANY ADDITIONAL DETAILS ABOUT THE VIRUS FOR THE TIME BEING.
THANK YOU FOR YOUR UNDERSTANDING!
CORONAVIRUS INFORMATION FOR HEALTHCARE PROFESSIONALS
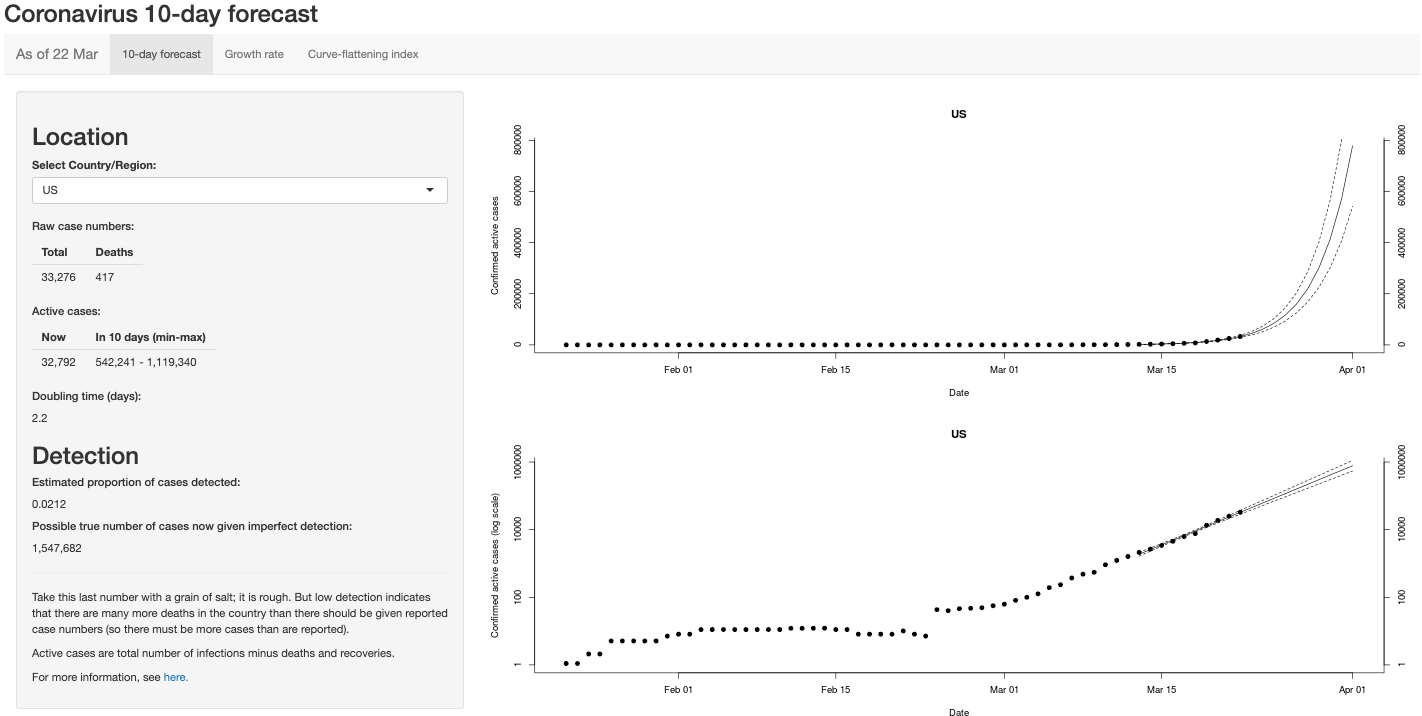
LAST UPDATE ON 03/25/2020.
THANK YOU FOR YOUR HARD WORK AND SACRIFICE!
Listen to hundreds of voice messages of gratitude from the public. You are appreciated and loved!
BACKGROUND
Coronaviruses (CoVs) cause a variety of diseases in mammals (including humans) and birds, including a wide range of enteric, hepatic, neurological diseases and respiratory tract infections. [1]
Although most Coronaviruses tend to only cause mild respiratory tract infections in Humans, a couple of them have gotten our attention over the past two decades. Both of these Coronaviruses were group 2b β-coronaviruses: Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) which caused SARS outbreak in 2002-2003 and Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) which emerged in 2012 in the Middle East. Both SARS and MERS had a high mortality rate especially in the elderly, but a relatively low transmission rate.
Current Coronavirus pandemic started in December 2019, with the first outbreak of a respiratory infection in Wuhan, China. It is due to Severe Acute Respiratory Syndrome Coronavirus 2” (SARS-CoV-2) which causes Coronavirus Disease 2019 (COVID-19). SARS-CoV-2 was not known until then.
- Enveloped, positive-sense single-stranded RNA virus
- ~125 nm sphere with club-shape spike projections on the surface making them look similar to a solar corona (hence the name)
- Realm: Riboviria, phylum Incertae sedis, order Nidoviales, family Coronviridae
- Coronaviruses are divided into 4 groups: alpha, beta, gamma and delta
Although most Coronaviruses tend to only cause mild respiratory tract infections in Humans, a couple of them have gotten our attention over the past two decades. Both of these Coronaviruses were group 2b β-coronaviruses: Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) which caused SARS outbreak in 2002-2003 and Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) which emerged in 2012 in the Middle East. Both SARS and MERS had a high mortality rate especially in the elderly, but a relatively low transmission rate.
Current Coronavirus pandemic started in December 2019, with the first outbreak of a respiratory infection in Wuhan, China. It is due to Severe Acute Respiratory Syndrome Coronavirus 2” (SARS-CoV-2) which causes Coronavirus Disease 2019 (COVID-19). SARS-CoV-2 was not known until then.